24 July 2025
Table of Contents
Introduction
Lung cancer is one of the deadliest cancers in the world, and small-cell lung cancer (SCLC) is its most aggressive form. It is difficult to treat and often comes back even after the treatment. A platinum-based chemotherapy called “Etoposide” is the first line of therapy given to the SCLC patients. Unfortunately, there are multiple patients who are resistant to this therapy and need another therapeutic regimen. Over the past 30 years, the second line therapy options for the treatment on SCLC have been limited where Topotecan is the sole standard of care treatment available in most of the countries. Now, there’s a new drug offering hope: Tarlatamab.
What is Tarlatamab?
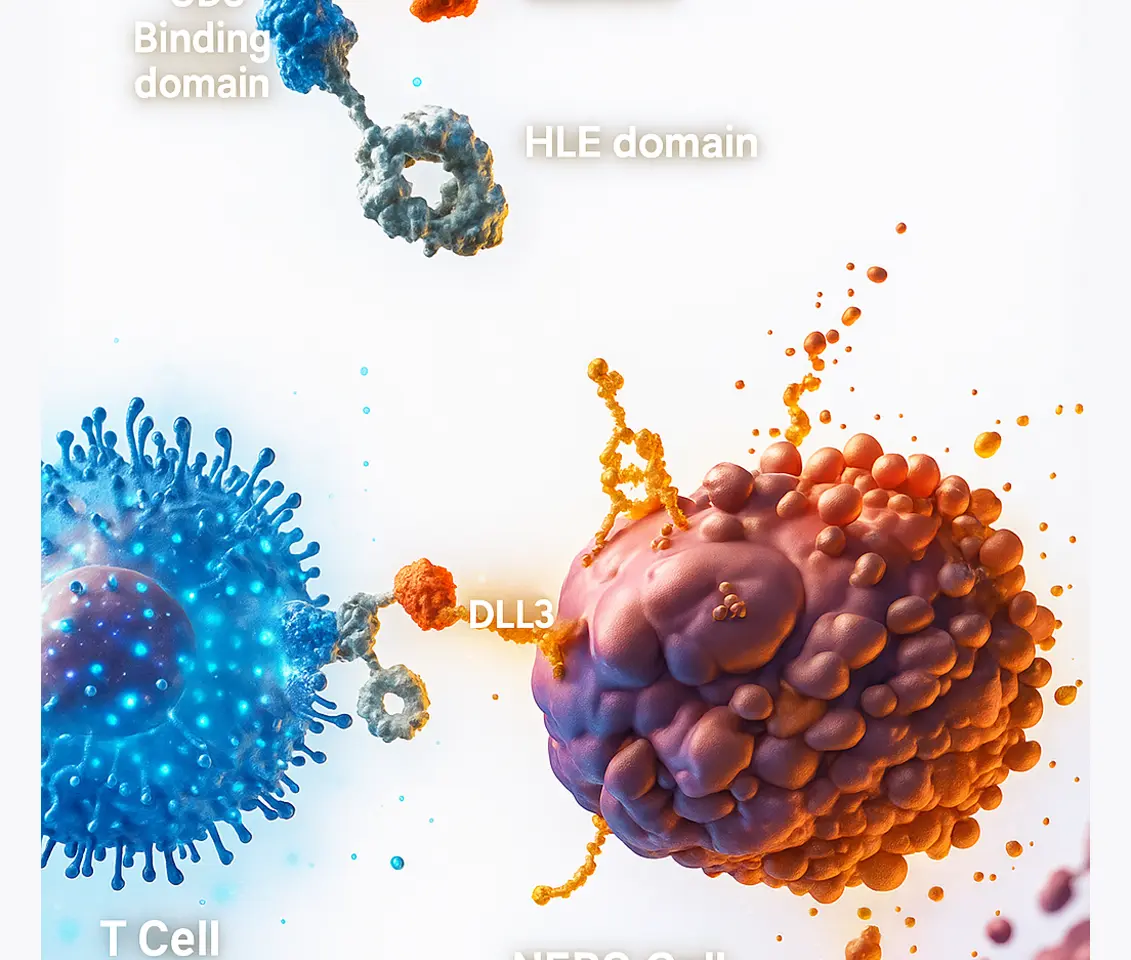
Tarlatamab is a new kind of medicine that helps the immune system find and destroy cancer cells. It targets a protein called DLL3, which is found mostly on small cell lung cancer cells but not on healthy cells. Tarlatamab works by acting like a bridge between immune cells (specifically T cells) and cancer cells and helps T cells to attaches to DLL3, which in turn recognize and kill the cancer cells.
How Was the Study Done?
Doctors ran a clinical trial to test how well Tarlatamab works and how safe it is. They gave the drug to over 500 patients with SCLC who had already tried chemotherapy but whose cancer had returned. The medicine was given through an IV drip every two weeks, and doctors watched closely to see: 1. If tumors shrank or stopped growing 2. How long the effect lasted 3. What side effects patients had
What Did the Study Find?
The results of this study were encouraging: 1. In almost 40% of patients responded to the treatment, meaning their tumors shrank or stopped growing for a period of time. 2. Some patients had long-lasting responses, with their cancer remaining under control for several months or more. 3. While there were some side effects (as with most cancer treatments), most of them were manageable. The most common were flu-like symptoms, fatigue, and low blood pressure.
How Does It Compare to Chemotherapy?
Chemotherapy is often the first treatment for SCLC and can work well at first. However, it attacks both cancer and healthy cells, leading to common side effects like hair loss, nausea, and low blood counts. When the cancer returns after chemotherapy, response rates to second-line chemo are usually below 25% and often short-lived. Tarlatamab, on the other hand, offers a more targeted approach, helping the immune system attack only the cancer cells. In this study, response rates were higher than typical second-line chemotherapy, and side effects were generally milder and more specific.
Why Is This Important?
For decades, treatment for small-cell lung cancer hasn’t changed much and the survival rates have remained low. A few of immunotherapy were successful in some patients, but the success rate is very low. Tarlatamab brings a new approach by combining the power of the immune system with precision targeting of cancer-specific markers. That’s a big step forward in a field where every bit of progress counts.
Takeaway
For people with relapsed SCLC, treatment choices are limited and often not very effective. Tarlatamab is a new kind of treatment that could change that. While more research is needed, it offers real hope for longer-lasting results with fewer side effects compared to traditional chemotherapy