07 June 2025
Rhabdomyosarcoma, a rare and aggressive cancer primarily affecting children and teenagers, presents a formidable challenge. This soft tissue cancer, often found in muscles, is notoriously difficult to treat, especially when it relapses after initial therapies like chemotherapy and radiation, which themselves carry significant side effects. However, groundbreaking research from scientists at the National Cancer Institute offers a beacon of hope through a novel approach in immunotherapy: CAR T-cell therapy.
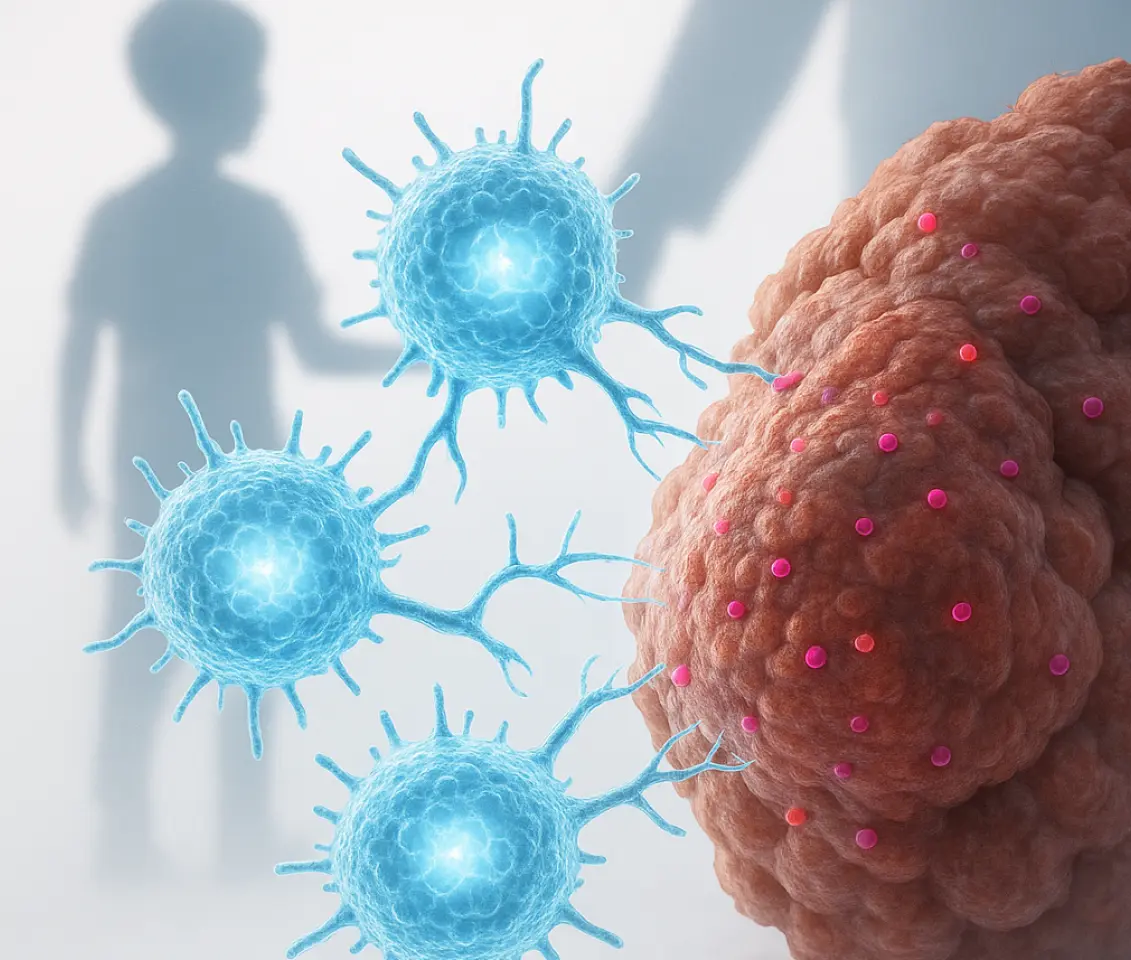
CAR T-cell therapy harnesses the body’s own immune system to combat cancer. It involves extracting a patient’s T cells (a type of white blood cell), genetically reprogramming them in the laboratory to specifically recognize cancer cells, and then reinfusing these “supercharged” cells back into the patient. Once reintroduced, these engineered T cells diligently seek out and destroy malignant cells. While this therapy has shown remarkable success in certain blood cancers, solid tumors like rhabdomyosarcoma have historically been much harder to conquer.
One key obstacle in treating solid tumors is their ability to “hide” from the immune system by altering the proteins on their surface. To overcome this, researchers have developed an advanced CAR T-cell therapy that simultaneously targets two distinct proteins: FGFR4 and CD276. These proteins are found in high concentrations on rhabdomyosarcoma cells, with minimal presence on healthy tissues. This innovative therapy is dubbed a BiCisCAR T cell, signifying its ability to recognize both targets concurrently within each engineered T cell.
Pre-clinical trials in mice models bearing human rhabdomyosarcoma have yielded highly encouraging results. This dual-targeting BiCisCAR T-cell therapy significantly outperformed single-target versions, demonstrating superior cancer cell killing, prolonged persistence within the body, and enhanced durability. Remarkably, even when fewer T cells were administered—mimicking real-world clinical conditions—the therapy remained effective and prevented cancer recurrence.
The exceptional potency of this approach stems from its unique internal “booster” system within each CAR T cell. It incorporates two different costimulatory domains: CD28 for a rapid initial attack and 4-1BB for sustained, long-lasting action. This dual-boost strategy enables the cells to proliferate robustly, maintain their strength, and continue their anti-cancer fight for extended periods.
Crucially, this treatment proved effective even when cancer cells attempted to evade the immune system by modifying their surface proteins. This is a monumental breakthrough, as immune “evasion” is a primary reason for the failure of CAR T-cell therapies in some cases.
While these findings are immensely promising, it’s vital to remember that this therapy is still in its nascent stages. Further research, particularly rigorous clinical trials in humans, are essential to confirm both its safety and effectiveness. Nevertheless, for families grappling with rhabdomyosarcoma, this research offers genuine hope that more intelligent, potent, and durable treatments are on the horizon.
In the ongoing battle against childhood cancer, this scientific advancement marks a truly bold and exhilarating step forward.