29 July 2025
Table of Contents
Introduction
Cancer treatment in India is rapidly evolving. And one of the most groundbreaking advances in recent years is CAR T-cell Therapy a treatment that uses your own immune cells to fight cancer. For patients whose cancers have returned or stopped responding to traditional treatments, this isn’t just hope it’s a second chance.
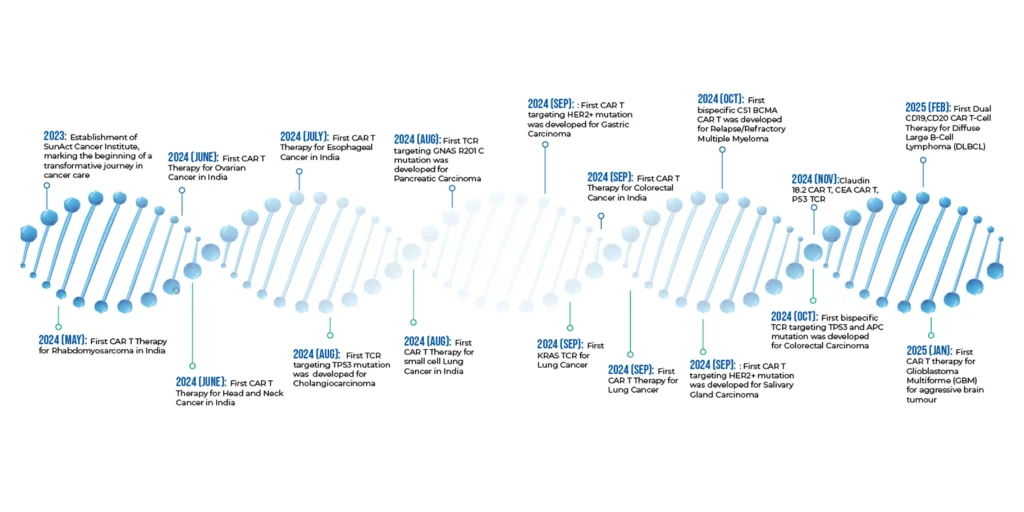
In Mumbai, SunAct has emerged as a national leader in this therapy. With India’s highest number of CAR T-cell and TCR therapies performed under one roof, SunAct is making world-class care accessible right here in India.
This blog explains everything you need to know from how the treatment works to where you can get it, how much it costs, and whether you’re eligible.
What is CAR T-cell Therapy?
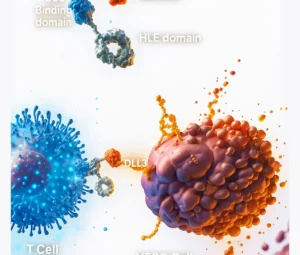
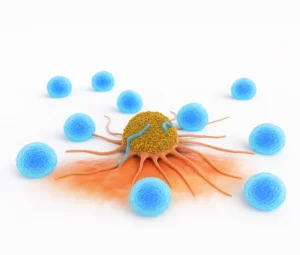
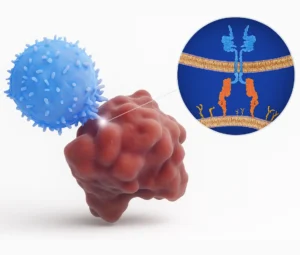
CAR (Chimeric Antigen Receptor) T-cell Therapy is a form of immunotherapy where doctors take your own T-cells (a type of white blood cell), genetically modify them in a lab so they can identify and kill cancer cells, and then infuse them back into your body.
Unlike chemotherapy, which affects both healthy and cancerous cells, CAR T-cells are highly targeted—reducing side effects and improving effectiveness.
Success rates:
Acute Lymphoblastic Leukemia (ALL): Up to 90% complete remission (Source: https://www.nature.com/articles/s41571-023-00754-1 )
Diffuse Large B-Cell Lymphoma (DLBCL): 50–60% complete remission (Source: https://www.mdanderson.org/publications/annual-report/annual-report-2017/CAR-T-c ell-therapy-shows-significant-remission-rates.html )
Multiple Myeloma: Durable responses observed with newer targets like BCMA and CS1 (Source: https://biovoicenews.com/first-patient-in-india-administered-bi-specific-car-t-therapy-f or-multiple-myeloma/ )
How CAR T-cell Therapy Works (Step-by-Step Process at SunAct)
Day | Step | Description |
-26 | T-cell Collection (Leukapheresis) | T-cells are collected from the patient’s blood. |
-5 | Manufacturing | T-cells are genetically modified to recognize cancer antigens. |
-3 | Patient Observation | Patient is prepared for infusion through observation and basic tests. |
0 | Lymphodepletion | Low-dose chemotherapy to make space for the CAR T-cells. |
+1 | CAR T-cell Infusion | Engineered T-cells are infused into the patient. |
+15 | First Efficacy Evaluation | Doctors evaluate the early response of the therapy. |
+30 | Discharge & Monitoring | If stable, patient is discharged and monitored remotely or via follow-ups. |
Who Can Benefit from CAR T-cell Therapy?
You may be eligible for CAR T-cell Therapy if:
- You’ve failed two or more previous cancer treatments.
- Your cancer tests positive for specific markers (like CD19 or BCMA).
- You’re physically stable (liver, kidney, and heart function within safe limits).
Note: Age isn’t a strict barrier, and SunAct has experience treating both younger and older patients.
Types of Cancers Treated with CAR T-cell Therapy at SunAct
Solid Tumours:
- Glioblastoma:
IL-13Rα2, HER2, GPC3, GD2 - Breast Cancer (including TNBC):
HER2, GPC3, Mesothelin - Ovarian Cancer:
HER2, Mesothelin, CEA - Lung Cancer:
Claudin 18.2, GPC3, GD2 - Pancreatic Cancer:
Claudin 18.2, Mesothelin - Colorectal Cancer:
CEA, Claudin 18.2, IL-13Rα2
Blood Cancers:
- Large B-Cell Lymphoma:
CD19 (ZUMA, JULIET studies) - Follicular Lymphoma:
CD19 (ELARA study) - B-ALL:
CD19 (ELIANA study) - Multiple Myeloma:
BCMA (KaRMMa, CARTITUDE studies)
Cost of CAR T-cell Therapy in Mumbai
In India, cellular therapies at SunAct begin at ₹35 lakhs.
What’s included:
- T-cell collection and lab processing
- Hospital stay (including ICU care if needed)
- Post-infusion support and medications
- Monitoring and specialist consultation
Financial Support at SunAct:
- CSR-funded therapy slots
- Tie-ups with health insurance partners
- Clinical trial-based subsidized options
Why Choose SunAct - Advanced Cancer Therapies?
- We have performed India’s Highest Number of CAR T-cell and TCR Therapies.
- We are a centre specializing in CAR T-cell therapy for both solid tumors and blood cancers.
- First in India to treat rare cancers like glioblastoma and salivary gland carcinoma.
- Led by pioneers like Dr. Vijay Patil and Dr. Ashay Karpe.
- Equipped with GMP-grade labs, ICU infrastructure, and Grade A clean rooms.
FAQs
Yes. Centers like SunAct follow global standards
with close monitoring for side effects.
You may qualify for re-infusion, TCR therapy, or
clinical trials.
About 3–4 weeks, including screening, manufacturing, infusion, and monitoring.
Not anymore. SunAct performs the complete process in-house—no international travel required.
At SunAct, we believe that advanced cancer care should be within reach for everyone. That’s why we actively support patients with insurance coordination, flexible payment options, and financial assistance wherever possible.
Cellular therapies at SunAct begin at ₹35 lakhs. For Bone Marrow Transplants (BMT), we offer some of the most cost-effective options in Mumbai—without compromising on quality or safety. We also collaborate with leading GMP-compliant BMT units across India to ensure standardized, high-quality care for every patient.
Final Thoughts
CAR T-cell Therapy is more than a treatment. It’s a new beginning for patients who have been told there are no options left. And with SunAct offering this advanced care in India, it’s accessible, safe, and proven.
If you or a loved one is fighting cancer, reach out to SunAct. Because in the fight against
cancer, no one should run out of options.Our team will reach out within 24 hrs of filing the
form.