20 June 2025
Mantle cell lymphoma (MCL) is a type of blood cancer that affects B-cells, which are important immune cells in our body. Unlike some other blood cancers, MCL is particularly aggressive and difficult to cure completely. While treatments like chemotherapy and newer targeted medicines can help control the disease, it often comes back, leaving patients and families searching for better options.
What Makes This Cancer So Challenging?
MCL has a specific genetic change that makes it grow quickly and resist many treatments. Even when patients respond well initially, the cancer frequently returns because it can “hide” from treatments by changing the proteins on its surface. This is where a revolutionary treatment called CAR-T therapy comes in, and now, scientists have made it even better.
Understanding CAR-T Therapy
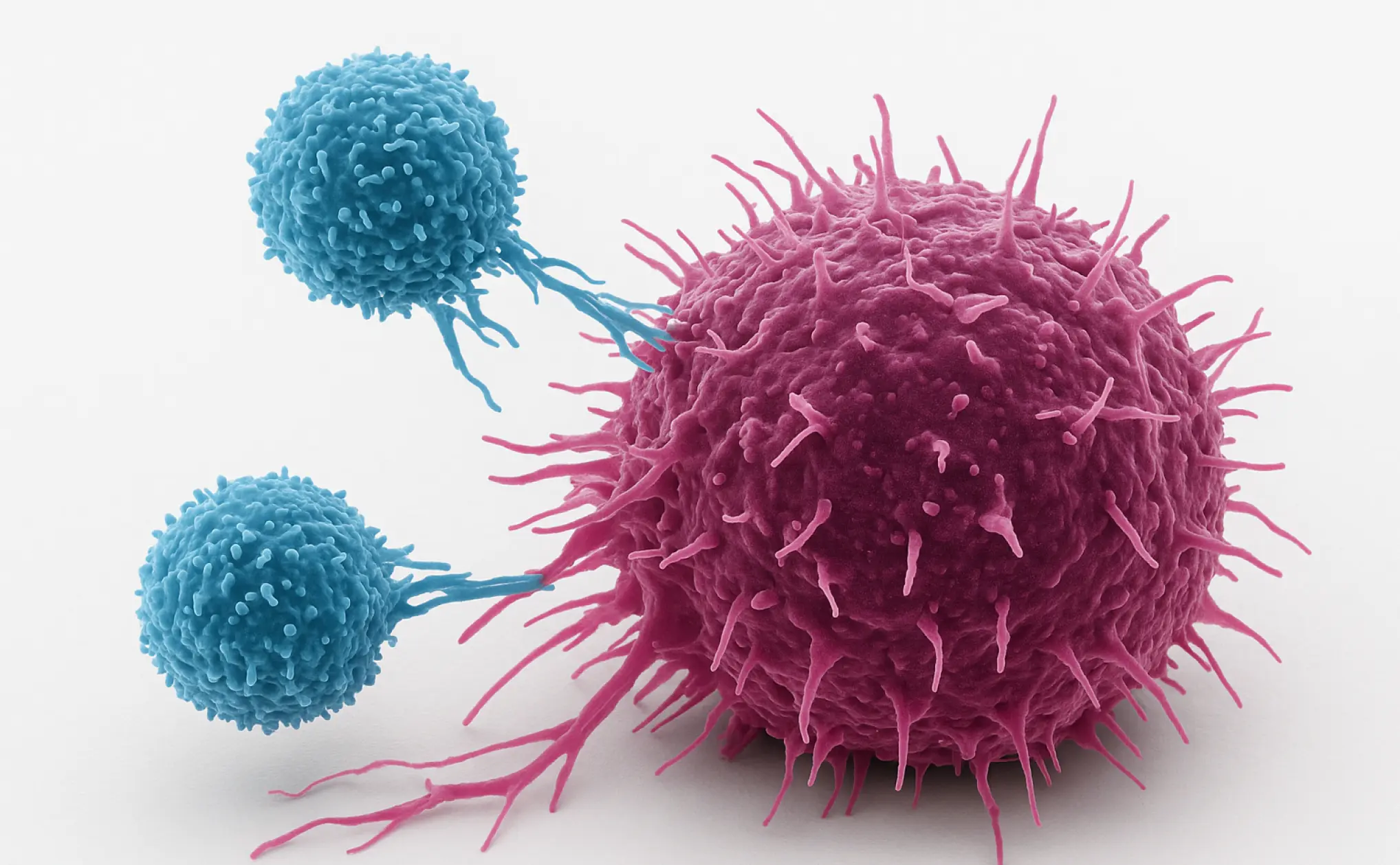
CAR-T therapy works like a precision-guided missile for cancer. Doctors take a patient’s own T-cells (the body’s natural cancer fighters) and reprogram them in a laboratory to better recognize and attack cancer cells. These “supercharged” T-cells are then given back to the patient through a simple infusion, similar to receiving a blood transfusion.
Traditional CAR-T therapy targets one protein on cancer cells called CD19. While this has helped many patients, some cancers learn to escape by hiding or losing this CD19 protein, causing the cancer to return.
The Innovation: Double-Target CAR-T
Scientists have now developed “bispecific” CAR-T cells that target two proteins at once—both CD19 and CD20. Think of it like having two different keys to the same lock. Even if the cancer tries to hide one protein, the CAR-T cells can still find and destroy it using the other target. This double-targeting approach significantly reduces the cancer’s ability to escape.
Promising Study Results
A recent study tested this new double-target CAR-T therapy in 17 patients with MCL that had returned after multiple treatments. The results were remarkable:
- 100% of patients responded to treatment, meaning their cancer shrank or disappeared
- 88% achieved complete remission, with no detectable cancer remaining
- Most patients who achieved complete remission stayed cancer-free for over a year
- The treatment was much safer than expected, with only mild side effects
What About Side Effects?
One of the biggest concerns with CAR-T therapy is side effects. The most common ones include fever and flu-like symptoms (called cytokine release syndrome) and sometimes confusion or neurological problems. In this study, all patients experienced only mild fever, and serious neurological side effects were rare and manageable. No patients needed intensive care within the first month.
The Manufacturing Advantage
This new approach also solved a practical problem. The researchers developed a faster way to make these CAR-T cells, taking only 8 days instead of the usual longer process. This means patients can start treatment sooner, which is crucial when dealing with aggressive cancers.
What This Means for Patients
For patients with relapsed MCL, this represents genuine hope. The combination of excellent effectiveness and manageable side effects suggests this could become a new standard treatment. The fact that patients who achieved complete remission with undetectable cancer (measured by very sensitive tests) remained cancer-free is particularly encouraging.
Looking Forward
While these results are extremely promising, this was a small study at a single hospital. Larger studies involving multiple hospitals are now planned to confirm these findings. Researchers are also exploring whether this approach could help patients earlier in their treatment journey, not just when other treatments have failed.
For patients and families facing MCL, discussing this option with your cancer care team could be worthwhile, especially if standard treatments aren’t working. This research represents the kind of scientific breakthrough that transforms cancer from a terminal diagnosis into a manageable or even curable condition.
The future of cancer treatment is becoming increasingly personalized and precise, and bispecific CAR-T therapy is leading that charge.