20 June 2025
Imagine if we could take a person’s own T-cells (a type of immune cells), train them to fight cancer and send them back into the body more strong, smarter, and ready for battle just like a fearless soldier in a battle-field. That’s exactly what CAR-T cell therapy does. It’s one of the most promising and personalized cancer treatments we have today. But to make these supercharged cells is a time taking process which often takes two to three weeks. Unfortunately, on the other hand, every day counts for someone battling aggressive cancer. Now, thanks to a new automated process, scientists can potentially make these cancer-fighting cells in just one day. Here’s what that means and why it matters.
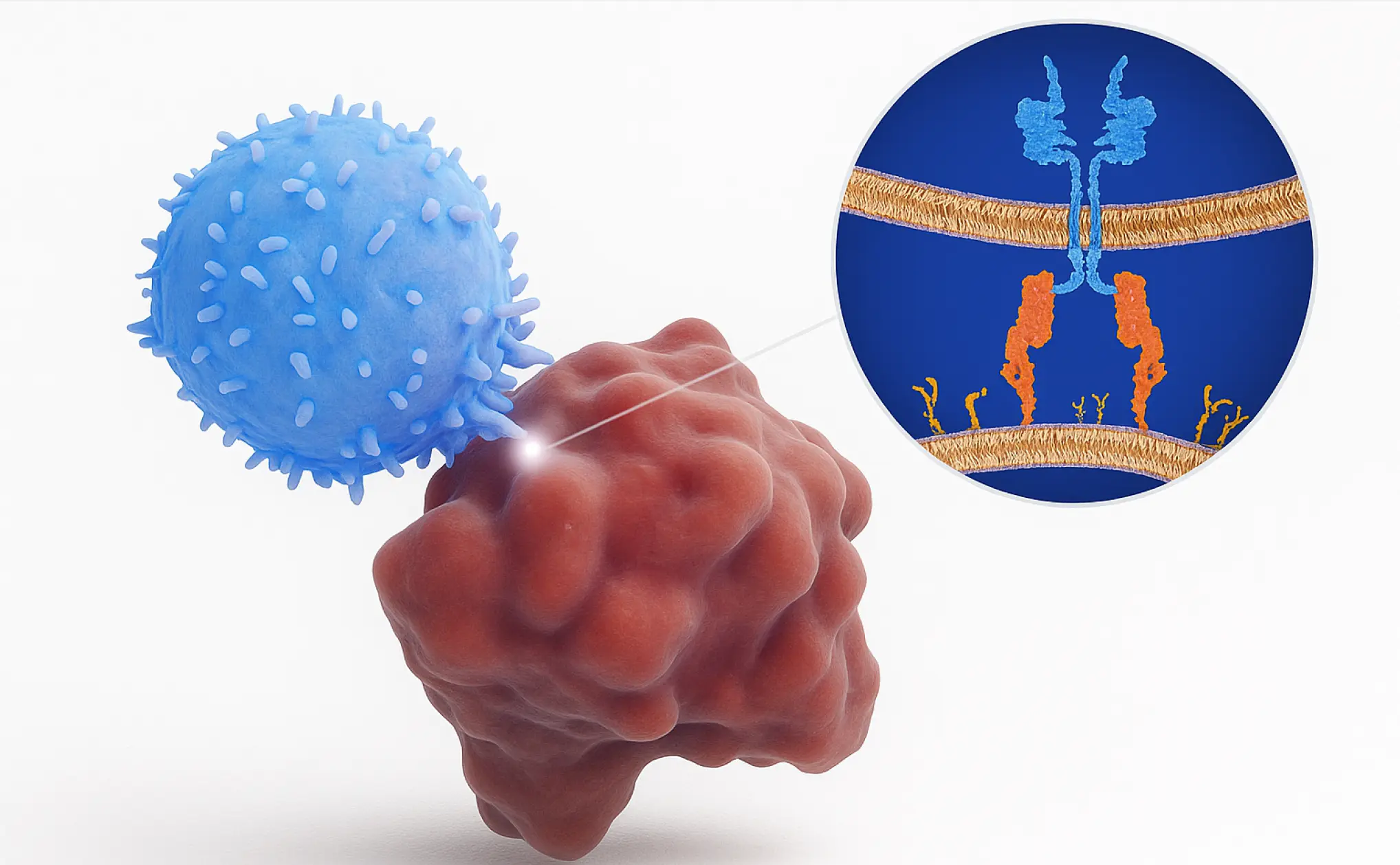
What Are CAR T Cells?
CAR T stands for Chimeric Antigen Receptor T cell. Basically, these are immune cells that are engineered in the lab to recognize and kill cancer cells.
The manufacturing process for these cells typically involves:
- Collecting T cells from the patient’s blood.
- Genetically modifying them to recognize cancer.
- growing millions of them, and then infusing them back to fight the disease.
Over the period of time, CAR-T therapy has completely revolutionized the cancer treatment but the complex lab work can be a big hurdle in the manufacturing time.
What’s New: FasT CAR‑T
Researchers developed a groundbreaking method called FasT CAR‑T, which slashes production time to just one day. Here, the T-cells are collected, genetically modified with any target receptor, and prepared in a single day. In this manufacturing process, the lengthy cell expansion is not required which reduces the manufacturing time just to one day. In comparison traditional CAR‑T, FasT CAR‑T cells have higher multiplication rates which in turn provides higher number of cells. Additionally, FasT CAR-T cells consist of younger populations of cells with higher central memory and stem-cell-like profiles which fight against cancer more vigorously.
In Humans Safety & Effectiveness
To check the efficacy of this newly formed drug, a clinical Phase I trial (25 children & adults with relapsed/refractory B‑ALL, ClinicalTrials.gov NCT03825718) was conducted and the results were very encouraging. The outcome of the study showed that the FasT CAR-T had very high response rate in patients with R/R B-ALL disease with no detectable disease. Most of the patients stayed disease-free for over 2 years (median remission 734 days). Interestingly, the relapse rate among these patients was very low.
In conclusion, these Fast CAR-T cells can be manufactured in just a day which is really crucial for patients with advanced cancer. Furthermore, these cells are younger, less exhausted T-cells which may expand more robustly and last longer in patients. Additionally, these cells came out to be more effective, even a small amount (30,000–150,000 cells/kg) led to complete remissions of the disease.
Here, this proof-of-concept study confirms FasT CAR‑T is feasible, safe, and promisingly effective.