05 July 2025
Table of Contents
Introduction
Cancer remains one of the most pressing global health challenges, with India witnessing a growing number of new diagnoses each year. While conventional treatments such as chemotherapy and radiation therapy have been the mainstay for decades, advances in biomedical research have ushered in a new era: cellular therapies. These therapies promise more precise, personalised, and potentially curative options.
This blog explores how SunAct – Advanced Cancer Therapies is transforming cancer care in India by offering cutting-edge cellular therapies. We outline the types of therapies available, their unique benefits, and how SunAct is making these innovations accessible and affordable across the country.
Understanding Cellular Therapies
What Are Cellular Therapies?
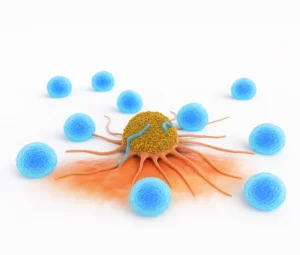
Cellular therapies utilize living cells, often from the patient, to treat diseases by enhancing the immune system’s ability to recognize and destroy cancer cells. These approaches offer targeted, durable responses and reduced toxicity compared to traditional therapies
Types of Cellular Therapies
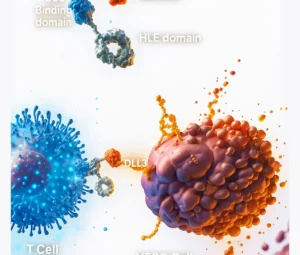
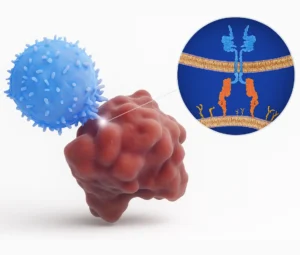
- CAR T-Cell Therapy: Patient’s T-cells are genetically modified to express chimeric antigen receptors
(CARs) that target cancer cells. - TCR Therapy: T-cells are engineered to recognize cancer-specific antigens presented via MHC
molecules. - TIL Therapy: Uses tumor-infiltrating lymphocytes harvested from the patient’s tumor, expanded in a lab, and reinfused to attack cancer.
- Gamma Delta T-Cell Therapy: Exploits a distinct subset of T-cells with innate anti-tumor
capabilities across various cancers. - Gene and CRISPR-Based Therapies: Employ gene editing tools to correct mutations or enhance
immune responses.
The Need for Advanced Cancer Treatments in India
India faces a rising burden of cancer, with many patients presenting at advanced stages. While standard treatments such as chemotherapy, radiation, and surgery remain effective and form the backbone of cancer management, there remains a need for additional, innovative options, particularly for relapsed, refractory, or resistant disease.
Cellular therapies offer a complementary approach that can extend survival, enhance disease control, and provide renewed hope even when standard treatments have been exhausted or have reached their therapeutic limit. These therapies represent a significant step forward in personalized and precision oncology.
SunAct: Pioneering Cancer Care
About SunAct
SunAct – Advanced Cancer Institute is a leading oncology center in India focused on next-generation treatments. Founded by distinguished oncologists Dr. Vijay Patil and Dr. Ashay Karpe, SunAct integrates clinical expertise with innovation to deliver transformative care.
Vision and Mission
SunAct is committed to democratizing access to cutting-edge therapies by making them affordable, evidence-based, and patient-centric. Their mission is to revolutionize the Indian oncology landscape through technology, research, and strategic partnerships.
SunAct’s Advanced Cellular Therapies
CAR T-Cell Therapy
SunAct offers both autologous (patient-derived) and allogeneic (donor-derived) CAR T-cell therapies for hematologic malignancies such as B-cell leukemias, lymphomas, and multiple myeloma. Research efforts are ongoing for their use in solid tumors.
TCR Therapy
TCR therapy is tailored to enhance T-cells’ recognition of tumor-associated antigens, enabling targeted elimination of solid tumors like melanoma, sarcoma, and lung cancer.
Tumor-Infiltrating Lymphocyte (TIL) Therapy
SunAct employs TIL therapy by extracting immune cells directly from tumors, expanding them ex vivo, and reinfusing them for a focused anti-tumor response—especially for melanoma, cervical, and other solid tumors.
Gamma Delta T-Cell Therapy
SunAct is conducting Phase 2 clinical trials of Gamma Delta T-cell therapy for aggressive cancers such as brain, breast, and lung cancers.
Gene and CRISPR Therapies
SunAct is exploring gene editing platforms such as CRISPR-Cas9 to correct oncogenic mutations and improve therapeutic precision in difficult-to-treat malignancies.
Comparative Overview of Cellular Therapies
| Therapy Type | Cell Source | Mechanism | Cancer Types | Personalization | Clinical Stage |
| CAR T-Cell Therapy | Autologous / Allogeneic | CAR-modified T-cells targeting tumor antigens | Hematologic cancers (e.g., B-cell malignancies) | Highly personalized | Approved, expanding into trials |
| TCR-T Cell Therapy | Autologous | TCR-enhanced T-cells recognizing MHC-bound tumor antigens | Melanoma, lung, sarcoma | Highly personalized | Investigational, trial use |
| TIL Therapy | Autologous (from tumor) | Expansion of tumor-resident lymphocytes | Melanoma, cervical, other solid tumors | Highly personalized | Clinical and investigational use |
| NK Cell Therapy | Autologous / Allogeneic | Innate immune attack via stress ligand recognition | Lymphoma, leukemia, some solid tumors | Semi-personalized | Emerging applications |
| Dendritic Cell Therapy | Autologous | Antigen-pulsed dendritic cells activating T-cell responses | Glioblastoma, prostate, melanoma | Personalized | Approved and investigational |
SunAct’s Nationwide Presence
SunAct is expanding to ensure equitable access across India:
- Thane (Mumbai): Flagship center at Tieten Medicity Hospital.
- Khar (Mumbai): Recently inaugurated Center of Excellence.
- Nashik: Specializing in advanced cellular therapies and bone marrow transplantation.
- Thodupuzha (Kerala): Partnership with Smita Memorial Hospital for advanced cellular therapies and bone marrow transplantation.
Affordability and Accessibility
SunAct’s mission includes reducing the cost barrier for Indian patients. Through optimized manufacturing and local partnerships:
- Cellular therapies at SunAct begin at ₹35 lakhs, a fraction of the cost in the US (₹2–3 crore).
- Support programs assist eligible patients with financial aid and insurance navigation.
FAQs
SunAct’s flagship center is in Thane, Maharashtra. Address: Tieten Medicity (Old Vedant Hospital), Ghodbunder Road, Kasarvadavali, Thane West, Maharashtra 400615 however we are also present in Khar, Kerala (Thodupuzha), Nashik and Kerala. You can check the contact section of our website.
We offer: - Cellular Therapies: CAR-T, TCR, TIL, Stem Cell Therapy - Conventional Therapies: Chemotherapy, Radiotherapy, Surgery - Targeted and Immunotherapies Supportive services include nutrition, counseling, and rehabilitation
Each case undergoes multidisciplinary review, with plans customized based on cancer type, stage, genetic profile, and overall health. Teleconsults are available.
Patients receive personalized follow-up care, survivorship planning, and rehabilitation services.
Yes. Patients meeting eligibility may enroll in cutting-edge trials under SunAct’s research wing.
Conclusion
SunAct – Advanced Cancer Therapies stands at the forefront of next-generation cancer treatment in India. By combining cellular innovation, global collaborations, and a vision of equitable access, SunAct is reshaping the oncology landscape—offering renewed hope for patients and families across the country.
Contact Information
📞 Phone: +91 86553 13412
📞 Toll-Free: 1800 202 2232
📧 Email: [email protected]
🌐 Website: www.sunactcancer.com