18 June 2025
Table of Contents
Introduction
Cancer is one of the leading causes of death worldwide. While treatments like chemotherapy, radiation, and surgery have helped many patients, they often come with severe side effects and are not always effective, especially when cancer spreads or comes back.
One of the newest and most exciting discoveries in this area is Gamma Delta (γδ) T Cell Therapy. In this blog, we’ll break down what Gamma Delta T cells are, how they work, how this therapy is different from others, the types of cancers it may treat, what SunAct offers, and what the future might look like.
Whether you’re a healthcare professional, researcher, or curious reader, this overview offers a crisp and clear look into one of cancer immunotherapy’s most exciting frontiers.
What are Gamma Delta (γδ) T cells?
Your immune system is like a security system for your body. It constantly looks for things that don’t belong, like viruses, bacteria, or cancer cells and tries to destroy them. One group of immune cells that does this job is called T cells.
Most T cells in your body are known as alpha beta (αβ) T cells. These cells usually need very specific signals like a fingerprint match, to identify a target. They rely on a system called MHC (Major Histocompatibility Complex) to find out if a cell is dangerous.
But there’s another, much rarer group of T cells called Gamma Delta (γδ) T cells. These are special because:
- They don’t need the MHC system to recognise danger.
- They can detect signs of stress or damage in cells.
- They act quickly, almost like first responders.
Gamma Delta T cells make up only about 1–10% of the total T cells in our blood. However, they’re powerful and play a unique role in patrolling for abnormal or dangerous cells.
How do they work against cancer?
Unlike regular T cells that need a very specific match to identify a target, γδ T cells can detect general danger signs, much like a smoke detector senses fire regardless of what caused it.
Here’s how they attack cancer:
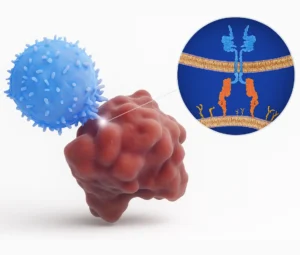
Spotting Danger
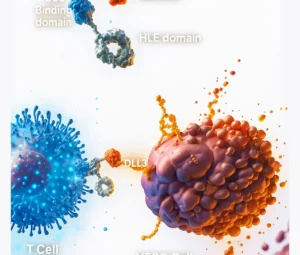
Gamma Delta T cells are great at sensing stress signals that cancer cells send out. Tumor cells often express proteins like MICA/B or phosphoantigens, which γδ T cells can recognize as “not normal.”
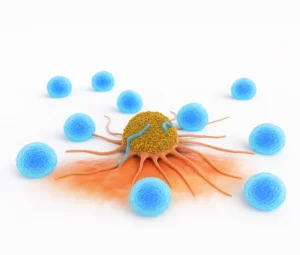
Killing Cancer Cells
Once they detect a cancer cell, γδ T cells release special substances like perforin and granzymes. These punch holes in the cancer cell and trigger it to self-destruct.
Sending Signals to Other Immune Cells
They release molecules like interferon-γ (IFN-γ) and tumor necrosis factor-alpha (TNF-α) to recruit other immune cells and boost the body’s overall immune response.
Working with Cancer Drugs
Gamma Delta T cells can also work alongside certain antibody-based cancer treatments. For example, they have a protein called CD16 that allows them to recognize and destroy cancer cells that are marked by therapeutic antibodies.
So essentially, gamma delta T cells are fast, flexible, and able to work both independently and as part of a team to fight cancer.
How does it work at SunAct?
SunAct is one of the first centers in India to offer this therapy through Phase 2 clinical trials. They use allogeneic γδ T cells, meaning the immune cells are donor-derived, processed outside the patient’s body, and infused into them after quality testing.
Key Steps:
- Donor cells are collected and activated to expand γδ T cells in the lab.
- These are then infused into the patient in controlled clinical settings.
- The γδ T cells identify and kill cancer cells while sparing healthy ones.
How is this different from other cancer treatments?
Feature | Gamma Delta T Cell Therapy | Chemotherapy | Radiation Therapy | Checkpoint Inhibitors |
Target Specificity | High – Targets stress markers on cancer cells | Low – Affects both cancer and healthy fast-growing cells | Moderate –Focuses on tumor area but may affect nearby healthy tissue | High –Reactivates immune response against specific tumors |
Damage to Healthy Cells | Minimal | High | Moderate | Low to moderate |
Personalization Needed | Often no – can use donor cells (off-the-shelf) | No | No | Sometimes, based on biomarkers (e.g., PD-L1) |
Side Effects | Usually mild (fever, fatigue) | Common and severe (nausea, hair loss, infections) | Localized (skin burns, fatigue, tissue damage) | Autoimmune-like effects (e.g., inflammation, organ damage) |
Type of Cancer Treated | Blood & solid tumors (experimental stage) | Most cancer types | Most solid tumors | Solid tumors with specific immune markers |
Immune System Involvement | Directly uses immune cells to fight cancer | Suppresses immune system | Minimal involvement | Boosts immune system response |
Treatment Duration | Short infusions or multiple doses | Weeks to months | Usually several sessions over weeks | Ongoing doses depending on response |
Availability | Limited (clinical trials) | Widely available | Widely available | Growing, but biomarker-dependent |
Gamma Delta T cell therapy is more versatile, less likely to harm healthy cells, and has lower risk of immune rejection compared to traditional T cell therapies. Also, it has the potential to be made into an “off-the-shelf” product that could be given to many patients without needing to make it from their own cells.
Safety Profile:
- Gamma Delta T cell therapies have generally exhibited favourable safety profiles
- Minimal occurrences of severe cytokine release syndrome (CRS) or neurotoxicity.
- Low-grade graft-versus-host disease (GvHD) observed in some patients, resolving without significant intervention.
- No dose-limiting toxicities reported in several early-phase trials.
Which types of cancer are treated at SunAct with this therapy?
Tumor Type | Ligands Identified (Expression %) |
Acute Lymphoblastic Leukemia | MICA/B (28–67%), ULBP1–3 (9–20%) |
Acute Myeloid Leukemia | MICA/B (0–75%), ULBP1 (16–50%), ULBP2 (4–64%), ULBP3 (16–100%) |
Bladder Carcinoma | MICA (70%) |
Brain Cancer | MICA/B and ULBP1–3 (90%) |
Breast Cancer | MICA/B, ULBP1–5 (35–100%) |
Cervical Cancer | MICA (20%), ULBP2 |
Chronic Lymphatic Leukemia | MICA/B (0–85%), ULBP1–3 (10–20%) |
Chronic Myeloid Leukemia | MICA/B (28–80%), ULBP1–3 (12–20%) |
Colorectal Cancer | MICA/B, ULBP1–5 (80–100%) |
Hepatocellular Carcinoma | MICA (60–100%) |
Lymphoma | MICA/B (20–44%), ULBP1–3 (12–20%) |
Melanoma | MICA/B (50%) |
Multiple Myeloma | MICA (10–60%), ULBP1–3 (0–34%) |
Neuroblastoma | MICA/B, ULBP1–3 (86%) |
Non-small-cell Lung Cancer | MICA/B, ULBP1–3 (20–30%) |
Ovarian Carcinoma | MICA/B, ULBP1–5 (50–97%) |
Pancreatic Cancer | MICA/B (68–89.3%) |
Prostate Cancer | MICA/B, sMICA/B (75–95%) |
Renal Carcinoma | MICA/B (>95%) |
Why choose SunAct?
SunAct isn’t just adopting this breakthrough, it’s helping bring it to India affordably. Here’s what makes them different:
- Experienced oncologists leading the clinical trial.
- Personalized treatment plans based on cancer type and stage.
- Focus on affordability, aiming to reduce the economic burden on Indian patients.
- Advanced infrastructure for safe cell therapy administration.
SunAct is not just testing the future of immunotherapy, it’s actively building it in India.
What can patients expect at SunAct?
- Eligibility screening before therapy.
- Infusion sessions that are short and non-invasive.
- Close monitoring by trained clinical staff.
- Potential for fewer side effects and improved quality of life.
- A role in shaping the future of cancer care through participation in clinical research.
What’s next for this therapy?
The future looks bright. If ongoing research continues to show positive results, we could see:
- Universal donor cells: Ready-to-use therapy that doesn’t need to be personalized for each patient.
- Longer-lasting treatments: Through gene editing or support from cytokines like IL-15.
- Combination therapies: Using γδ T cells along with vaccines, checkpoint inhibitors, or traditional drugs.
- Wider access: Moving from experimental settings to real-world hospitals and clinics.
The hope is to make gamma delta T cell therapy a standard option for many types of cancer, especially for patients who have no other options left.
FAQs
GDT therapy is typically recommended for patients with advanced cancers who have not responded to conventional treatments like chemotherapy or immunotherapy. A thorough medical evaluation is required to determine eligibility.
Cytokine Release Syndrome (CRS): May occur, leading to symptoms such as fever, fatigue, and low blood pressure.
Immune-Related Side Effects: Possible inflammation or autoimmune reactions.
Other Side Effects: May include infusion reactions, infections, and general fatigue.
Clinical trials have shown that patients with advanced or recurrent cancers who undergo GDT therapy experience significant tumor shrinkage and prolonged survival. The therapy has particularly shown efficacy in cancers like Blood cancer and lung cancer.
Conclusion
Gamma Delta T Cell Therapy is still new, but it’s real, it’s working, and it’s available at SunAct. As one of India’s pioneers in this domain, SunAct – Advanced Cancer Therapies offers patients a chance to participate in next-generation treatment today, not tomorrow.
If you or a loved one is exploring options for advanced cancer care, SunAct’s team is ready to help you make an informed decision.
🔗 Learn more or apply for the clinical trial at:
👉 https://sunactcancer.com/gamma-delta-t-cell-therapy